Research
Adaptation to the Host Environments
Our work on adaptation to the host focuses on nutrient uptake and metabolism, the connection between cell wall and stress responses, and pneumococcal-influenza co-infections.
Cell Wall Biosynthesis, Translational Fidelity & the Stringent Response
Collaboration with Dr. Sergio Filipe at Universidade NOVA de Lisboa and Drs. David Roper and Adrian Lloyd at the University of Warwick.
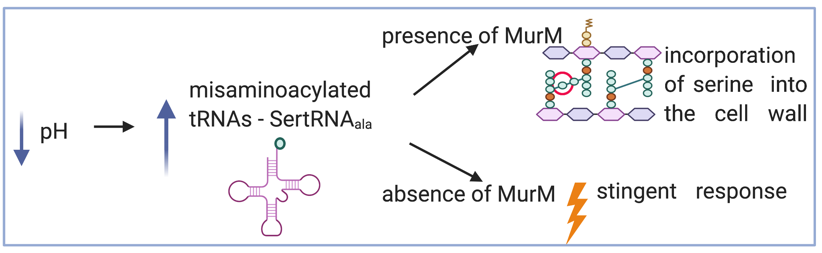
When exposed to stresses bacteria activate the stringent response pathway, a stress response that re-configures bacterial metabolism to ensure survival. We found that the stringent response can be activated by increases in misaminoacylated tRNA (mostly toxic seryl-tRNAAla) which accumulates at low pH. In addition, we found that MurM, a cell wall biosynthesis enzyme, displays a strong preference for amino acids from the misaminoacylated seryl-tRNAAla, compared to the correctly acylated seryl-tRNASer or alanyl-tRNAAla. Thus, when MurM removes serine from misaminoacylated seryl-tRNAAla it serves as a gatekeeper of the stringent response pathway. Further, accumulation of seryl-tRNAAla leads to errors in translation, such that ability of MurM to deacylate these molecules contributes to translation fidelity. In the absence of MurM, accumulation of mischarged tRNAs triggers the stringent response, premature entry into stationary phase, and subsequent autolysis. In most domains of life, the pathological consequences of misaminoacylated tRNAs are mitigated by AlaXp, an enzyme that deacylates mischarged tRNAAla. Pneumococcus, and multiple Gram-positive bacteria, do not encode AlaXp. This work supports a model where MurM is an alternative evolutionary solution to the challenge of misaminoacetylation. These findings implicate cell wall synthesis in the survival of bacteria as they encounter unpredictable and hostile conditions in the host. In this manner, environmental stresses may be reflected as variations in cell wall composition. The association between cell wall synthesis and translational fidelity is likely to be active in many other Gram-positive pathogens, given the distribution of MurM homologues and conservation in cell-wall cross-bridges.